Inspecting vials and ampoules of vaccines and diluents
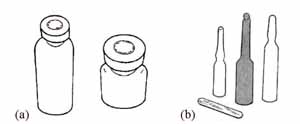
Vaccines and diluents are supplied in either a vial or an ampoule. A vial is a glass bottle with a thin rubber membrane across the top, which is held in place by a metal or plastic cap (Figure 4.5a). An ampoule is a sealed sterile glass or plastic bottle with a thin ‘neck’ (Figure 4.5b). The ampoule has to be broken open at the neck before the vaccine (or diluent) can be withdrawn. Note that some injectable vaccines are supplied in single-dose vials, and some contain more than one dose. You will learn how to use multi-dose vials in Study Session 7.
Vaccines (e.g. BCG) that are sensitive to light are supplied in dark glass vials or ampoules.
Vials and ampoules should be carefully inspected for damage or contamination prior to use. The expiry date printed on the vial or ampoule, or the box they came in, should be checked. The expiry date gives the last day of the month that the vaccine or diluent can be used, unless otherwise stated on the package labelling. Expired vaccines and diluents should never be used. You will learn more about stock control to avoid wastage in Study Session 5.
Check the vaccine vial monitor (VVM), which is a label that changes colour when the vaccine vial has been exposed to heat over a period of time. The VVM enables you to check if the vaccine has not passed the discard point due to heat exposure. It cannot tell you if the vaccine has been damaged by freezing. You will learn more about this, and other components of the ‘cold chain’ for preserving vaccines, in Study Session 6.
What is the correct temperature for storing vaccines supplied as liquids?
It is between 2oC and 8oC, as you learned in Study Sessions 2 and 3.