Rotavirus vaccine (RotarixTM)
Rotaviruses are the leading cause of severe diarrhoeal disease and dehydration among children in developed and developing countries. Globally, more than 125 million cases and up to 610,000 deaths annually are due to rotavirus diarrhoea. According to WHO report, 20% of under five years child deaths from diarrhoeal diseases worldwide are due to rotavirus infection - most of them in countries like Ethiopia. Rotavirus vaccines become available as part of the routine EPI in Ethiopia.
Dosage and schedule of rotavirus vaccine (RotarixTM)
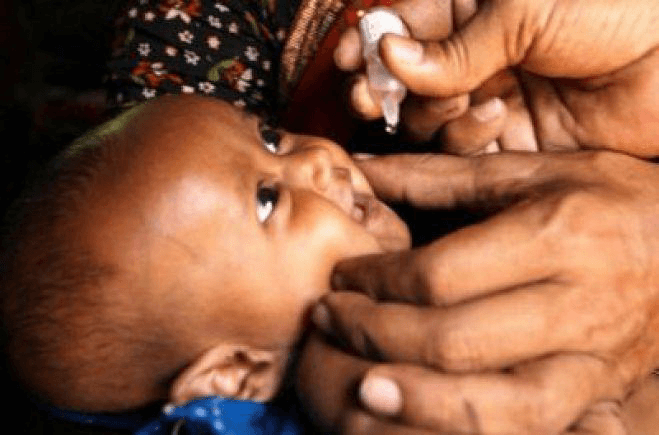
Two new live attenuated rotavirus vaccines have been licensed for use in the routine immunisation programme. They are both given orally to infants as drops into the mouth. The vaccine chosen for the EPI in Ethiopia is known by its brand name RotarixTM. It is a liquid suspension vaccine, supplied in single-dose "squeeze-tube" vials. RotarixTM is given in oral doses, each of 1.5 ml, at the following time intervals:
During administration
The vaccine should be inspected visually for any foreign particulate matter and/or abnormal physical appearance. In the event of either being observed, discard the vaccine. The vaccine should be well shaken before use. It is important to open the vaccine tube correctly to prevent the small nozzle being dropped, and possibly inhaled by the child.
Schedules of the vaccine
- First dose at six weeks of age, but no later than 12 weeks.
- Second dose at least four weeks after the first dose.
The two-dose schedule should be completed within 16 weeks, but no later than 24 weeks of age. Note that the ideal schedule is to give the first dose of RotarixTM to all infants at six weeks of age at the same time as giving Penta1 and OPV1, and give the second dose at ten weeks at the same time as Penta2 and OPV2.
Storage and effectiveness of RotarixTM
RotarixTM is a freeze-sensitive vaccine which must be stored in the refrigerator at a temperature of between +2ºC to +8ºC. It is a very safe vaccine, which provides 90-100% protection from severe rotavirus disease to fully immunised infants, and 74-85% protection against rotavirus diarrhoea of any severity. You will receive instructions on the contraindications for giving rotavirus vaccine and the management of possible adverse events following immunisation when RotarixTM is introduced into the EPI in Ethiopia.
Like all vaccines, the rotavirus vaccine can cause side effects, but they're mild and short-lived.
Common side effects of the rotavirus vaccine
Babies who have taken the vaccine sometimes become restless and irritable, and some may develop mild diarrhoea.
Contraindications of RotarixTM
- Reported hypersensitivity following previous administration of the same vaccine, or reported hypersensitivity to any of the vaccine components will be a contraindication.
- Prior history of intussusceptions.
- Subjects with uncorrected malformation of GI tract that would predispose to intussusceptions.
- Severe immune deficiency.
- The vaccine should not be re-administered after regurgitation or spitting out a dose.
Precautions: Vaccination in infants with ongoing severe gastroenteritis or severe febrile illnesses should be postponed until the child completely recovers. The presence of minor infections, however, is not a contraindication for vaccination.