Water quality assessment
As you know, water can be polluted by chemical or biological contaminants and the water may be harmful to humans when consumed. There are many analytical methods used to test for the presence and concentration of possible pollutants. You are not expected to carry out microbiological and chemical tests of drinking water but it will help you if you understand the principles.
If at all possible, drinking water should not contain any pathogenic microorganisms. It would be very difficult and time-consuming to test for all the possible pathogens. The source of the pathogens is usually human faeces; therefore, tests have been devised that detect the presence of faecal contamination. If faecal contamination is found, this indicates that pathogenic organisms may be present.
The most widely used tests for faecal contamination are total coliforms, faecal coliforms and Escherichia coli (E.coli). Coliforms are a group of bacteria found in human and animal faeces and also in soils and some other natural environments. ‘Total coliforms' includes all bacteria in this group. The presence of ‘total coliforms' indicates contamination of some sort but, because of their relatively wide distribution, cannot be used to confirm if the contamination is from faeces.
Faecal coliforms are a sub-set of total coliforms and, as the name suggests, are typically found in faeces. However, even this group includes some species that are not necessarily faecal in origin. E.coli is a type of faecal coliform bacterium that is found only in faeces of humans and other warm-blooded animals. If E.coli is present in a water sample, this indicates faecal pollution and the possible presence of pathogenic types that often occur in the intestines as well; the absence of E.coli from a sample shows that the chances of faecal contamination of the water, and therefore of pathogens being present, are negligible. Thus the presence or absence of E.coli in a water sample provides an important indicator of pollution and public health. However, it is important to realise that E.coli is only an indicator and its absence cannot give complete assurance that water is safe. Some pathogens such as Giardia, Entamoeba histolytica and some viruses are more resistant to disinfection than E.coli; therefore, the absence of E.coli will not necessarily mean that water is totally free from other organisms.
Although the great majority of health-related water quality problems are the result of bacteriological contamination, chemical contamination of water sources can also cause serious health problems. For example, the presence of nitrate and nitrite in water may result from the excessive application of fertilisers or from seepage of wastewater into surface water and groundwater. Fluorosis is a common problem in children living in areas of high levels of naturally occurring fluoride in the water which can lead to mottling of children's teeth, skeletal fluorosis and crippling.
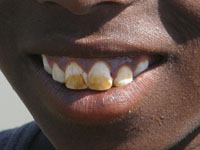
Some health effects may occur as a result of specific chemical deficiencies in the diet, of which water forms a part, for example, goitre caused by iodine deficiency and dental caries resulting from low fluoride intake.
Turbidity, colour, taste and odour (smell), whether of natural or other origin, affect people's perceptions of water. As you know water should be free of tastes and odours that would be unpleasant to the majority of people. In extreme cases, people may avoid water that does not look or taste good – even if it is otherwise safe – in favour of more pleasant looking and tasting water that may actually be contaminated. Colour in drinking water occurs due to the presence of organic matter and metals such as iron and manganese. Odour in water is due mainly to the presence of organic substances. Taste is the combined perception of substances detected by the senses of taste and smell. Changes in the normal taste of a municipal water supply can be important as they may signal changes in the quality of the raw water source or deficiencies in the treatment process. Onsite testing is essential for the determination of turbidity and residual chlorine, which change rapidly during transport and storage.