Methods of household treatment of water
What is sedimentation?
Sedimentation is the removal of suspended solids through the settling of particles.
Just as in the water treatment works, if the water is turbid (muddy), then giving it time to settle (become calm) helps in sedimentation. This process can be assisted by adding a coagulant that encourages the formation of larger, heavier particles (flocculation) that then settle easily to the bottom of the container and make the water clear. At the household level, natural coagulants can be used such as the crushed, dry seeds of the Moringa fruit, shown in Figure 10.3 (Davis and Lambert, 1995). Moringa fruit comes from Moringa trees, which grow in tropical countries

Figure 10.3 Fruits of Moringa oleifera.
Can you name one of the coagulants used in large-scale water treatment?
As you learned in Study Session 5, Section 5.2.3, aluminium sulphate and ferric chloride are used as coagulants in large-scale water treatment.
After the suspended particles have settled, the clear water at the top can be carefully poured into another container for further processing. The three-pot method is one method of sedimentation.
The three-pot method
The three-pot method (Figure 10.4) is a means of reducing dirt and micro-organisms in water, by storing the water in a container, allowing the dirt to settle, and moving the cleaner water to different containers over time. Water in a container should be allowed to settle for a day before it is decanted into the next container. Only water from Pot 3 should be consumed. The three-pot system is low-cost, easy to use and is something people can do themselves. However, it does not totally remove disease-causing micro-organisms, so some method of disinfection is still needed to remove the risk of disease completely.
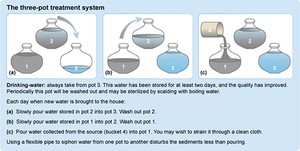
Figure 10.4 The three-pot water treatment system (IFRCRC, 2008).
Filtration
Filtration is another method of removing suspended particles and is also relatively easy. There are several different methods of filtration that can be used in the home.
Cloth filtration
Cloth filtration (Figure 10.5) is cheap, easy to carry out and a common water treatment technique. Pouring turbid water through a piece of fine, clean cotton cloth will remove larger contaminants and a certain amount of suspended solids. It is better to use a used, rather than new, piece of cloth.
Why do you think a used, washed piece of cloth is better for filtration?
Once cloth has been washed several times, the gaps between the fibres it is made from are smaller and therefore better for trapping any solid matter.

Figure 10.5 Cloth filtration.
The steps in cloth filtration are as follows:
- Use a large cloth, preferably made of finely-woven cotton. Fold the cloth at least four times so that there are multiple layers of fabric, and place this over the opening of the storage vessel.
- The cloth, once folded, must be big enough to easily cover the opening of the receiving water container.
- Place the cloth over the mouth of the container.
- Fasten the cloth securely around the rim of the opening, using string. If reusing the cloth, always use the same side up each time.
- Pour the water through the cloth, into the container.
- Wash the filter cloth after each use, with a final rinse using cloth-filtered water, and then leave the cloth in the sun until it is dry.
- Clean the cloth regularly using detergent, and use a new piece of cloth as soon as there are any visible tears or holes.
- Always keep filtered water separate from non-filtered water.
Household sand filtration
A household sand filter can usually be made from locally available and inexpensive materials like clay pots or barrels. They are simple and easy to use. One such system consists of a pot and a storage vessel (Figure 10.6).
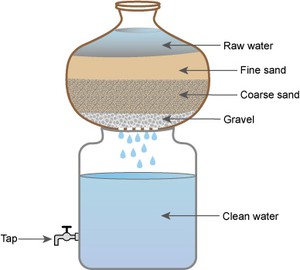
Figure 10.6 A household sand filter system, comprising a pot and a storage vessel.
The details of the system are as follows:
- The bottom of the pot is perforated (has tiny holes in it).
- The pot contains layers of gravel (about 5 cm deep), coarse sand (about 5 cm deep) and fine sand (about 10 cm deep).
- Water is poured in at the top and, as it passes through the layers, any particles within it are filtered out.
- Clean water drips into the storage container.
- The storage container should have a tap to enable the clean water to be drawn out easily and safely.
- The sand and gravel should be changed when the rate of filtration starts to slow; at a minimum it should be changed every two to three months.
Ceramic filtration
For ceramic filtration, a water filter in the form of a ceramic pot can be made using clay, sawdust or rice husks, and a plastic bucket. The pot is made by mixing clay with the sawdust or rice husks, forming it into a flowerpot shape and then firing it in a kiln. The sawdust or rice husks burn away, leaving tiny pores in the ceramic through which water can be filtered. The small pore size of the ceramic material traps the particles and most micro-organisms.
To use the filter, the pot is inserted into a container so that its lip prevents it from slipping into the container (Figure 10.7). The raw water is then poured into the ceramic pot. Cleaned water percolates out of the pot and is collected in the container below. A tap on the container allows water to be drawn out.
The filter is cleaned by gently scrubbing the surface, and it is recommended that the filter be replaced every 1–2 years, as fine cracks not visible to the naked eye may have developed.
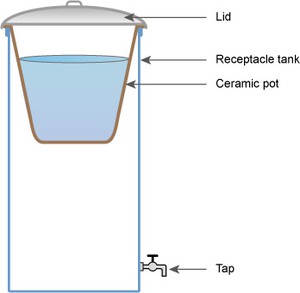
Figure 10.7 Ceramic pot method of water filtration.
Disinfection
Disinfection is the final stage of water treatment in the home. All water treated using one or more of the previous steps will need to be disinfected to ensure that all pathogens are killed. The three common methods of disinfection are described below.
Boiling
Boiling drinking water is a simple way of killing pathogens and is suitable for use at household level. Boiling destroys pathogens such as bacteria and viruses, and any parasite ova (eggs) present in water. The water should be heated until large bubbles come continuously to the surface of the water (referred to as a ‘rolling boil’), for at least 5 minutes. This has been shown to inactivate cholera and Shigella organisms (Luff and Clarke, 2006). If the location of the site is at a high elevation (as in Addis Ababa) the water should be boiled for longer. Boiling will make the water taste flat but this can be remedied by shaking the water in a clean bottle, or by adding a pinch of salt to one litre of water. If the water is turbid with particles, it should be filtered before boiling. Water should be boiled, cooled and stored, all in the one container and consumed within 24 hours to prevent re-contamination.
Can you think of disadvantages associated with boiling?
Fuel has to be obtained, and scalding accidents can occur if people are careless. But it is still the simplest way of ensuring that water is safe to drink.
Solar disinfection (SODIS)
Solar disinfection, known as SODIS, is a water treatment method that uses ultraviolet (UV) radiation and high temperature from the sun to destroy micro-organisms in water. The technique requires only some clear plastic bottles (without labels) and sunlight (Figure 10.8).

Figure 10.8 Plastic bottles full of water being laid out in the sun for disinfection.
The steps to take for SODIS are shown in Figure 10.9. In Step 5, the bottles are placed on a corrugated iron sheet (often used as roofing material) which is painted black so that it retains heat from the sun, speeding up the rate of heating of the water.
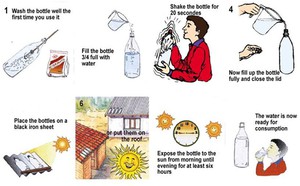
Figure 10.9 The procedure for solar disinfection of water (SODIS).
Chemical disinfection using chlorine
Chlorine solution, also known as sodium hypochlorite or bleach, is the most affordable and widely available chemical for household water treatment. Typically the procedure is to add a capful of chlorine solution to water in a 20–25 litre storage container, then stir and wait for 30 minutes. As you learned in Study Session 5, this period of time is referred to as the contact time. After this, the water can be used.
Chlorination is effective if the water is not turbid. If the water is turbid, micro-organisms can shelter within the particles and escape the effect of the chlorine. Solids should therefore first be removed by sedimentation or filtration. It is important that some residual chlorine remains in the water at the time it is used, so that it stays safe to drink. A minimum concentration of 0.5 mg l–1 is recommended; this will kill any organism that enters the water later.