Types of water pollutant
There are many different types of water pollutant and the following sections describe those that are most commonly found.
Sediments and suspended solids
Sediments and suspended solids consist of fine particles of mostly inorganic material. Inorganic material is derived from non-living sources and includes mud, sand and silt washed into a river as a result of land cultivation, construction, demolition and mining operations, where these take place. One of the most common sources of suspended solids and sediment is soil erosion, where the soil is washed away into rivers by rainwater run-off. The presence of solid particulate material suspended in the flowing water is the reason why many rivers look brown in colour, especially in the rainy season. The particles are called suspended solids while they are carried (suspended) in flowing water and sediments when they settle to the bottom. Large quantities of suspended solids may reduce light penetration into the water, which can affect the growth of plants. Sediments may even suffocate organisms on the river bed.
Organic matter
Organic matter, such as human and animal wastes, is derived from living organisms. As organic matter decomposes, it removes oxygen from the water and this can have a damaging effect on fish and other aquatic organisms that are sensitive to poor water quality. Box 4.1 explains this process. If a large quantity of organic matter is present in surface water, this can lead to anaerobic conditions. (Anaerobic means without oxygen, as opposed to aerobic, which means oxygen is present.) In this situation many aquatic organisms are unable to survive and the water will be stagnant and smell unpleasant.
Box 4.1 Oxygen in water
Many aquatic (water-living) organisms depend on oxygen dissolved in the water to survive. Aquatic animals include fish, amphibians and many invertebrate species such as insect larvae, snails and worms. Their supply of oxygen in the water is maintained from atmospheric oxygen in the air above the water and from oxygen produced by green aquatic plants by the process of photosynthesis, the process by which plants convert light energy into chemical energy, while taking in carbon dioxide from the atmosphere and producing oxygen. Fast-flowing, turbulent water will be aerated (gain oxygen) more than still water because the turbulent flow will entrain more oxygen.
If organic pollutants such as human and animal wastes are released into a water body, bacteria will use the waste as food and break it down into simpler, less harmful substances. As they do this, aerobic bacteria will use up the dissolved oxygen from the water. This is called deoxygenation. If the degree of organic pollution is high, then all the oxygen from the water may be used up, leading to anaerobic conditions.
This is unlikely in a river where the water is moving but can happen in lakes or slow-flowing channels. Inorganic solids, such as mud and silt, do not have this effect because they are inert (stable and inactive) and cannot be used as food by bacteria.
Biological pollutants
You have already learned about biological pollutants in Study Session 2. These are the infectious agents (bacteria, viruses, protozoa and helminths) that are harmful to humans and other forms of life. Biological pollutants may get into water with dust from the air as rain falls but the most likely source is from water that is contaminated with human and animal wastes.
Plant nutrients
Nitrates and phosphates are common pollutants generated from residential areas and agricultural run-off. They are usually associated with human and animal wastes or fertiliser that has been washed into surface water bodies by rain. Nitrates and phosphates are plant nutrients, so they stimulate plant growth. If present in large quantities, they can encourage excessive plant growth in the water causing the phenomenon known as an algal bloom, which means a sudden increase in the population of microscopic algae (simple plants). There may also be an increase in larger plants such as the invasive water hyacinth. When the increased population of aquatic plants dies, the decay of the organic plant material by bacteria can cause deoxygenation of the water, resulting in the death of other organisms such as fish. If a water body has high nutrient levels it is said to be eutrophic and the process is known as eutrophication. Figure 4.3 illustrates the process.
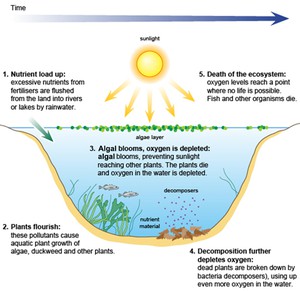
Figure 4.3 The eutrophication process.
Can you think of a reason why eutrophication is more likely to be a problem in lakes than in rivers?
Because flowing water in a river will disperse the nutrients; in the still water of a lake, the nutrients will accumulate.
In Ethiopia, many private and corporate farms use huge amounts of chemical fertilisers. As a result, eutrophication is becoming a major problem (Zinabu et al., 2002), affecting many water sources (Figure 4.4).

Figure 4.4 Lake Hawassa, in the Rift Valley, south of Addis Ababa, is suffering from eutrophication.
Other chemical pollutants
Heavy metals such as arsenic, copper, lead, mercury and cadmium are chemical pollutants that may be found in lakes, rivers and groundwater. These heavy metals can harm aquatic organisms and humans. Farmers who use river water polluted by urban wastes for irrigation in the cultivation of fruits and vegetables may find their crops affected by the accumulation of these chemicals. (You will look at a case study on this later on in this study session.)
Pesticides include insecticides, herbicides and fungicides. There are several thousand different types in use and almost all of them are possible causes of water pollution. Pesticides such as DDT (dichlorodiphenyltrichloroethane), malathion, parathion and others have been sprayed in the environment for long periods of time for the control of disease vectors such as mosquitoes and other pests.
Heavy metals and some pesticides are particular problems because they are persistent in the environment, meaning they do not break down and their effects continue over time, even long after their use may have stopped.
Another problem can be acidity. If water becomes acidic or alkaline, beyond normal limits, this will have a damaging effect on aquatic organisms. Acidity and alkalinity of water are determined by measuring its pH. A pH value below 7 is acidic and above 7 is alkaline. Acidic water is not only harmful to life but is also corrosive and can damage pipework in water distribution systems.